Results from low-cost COVID-19 study announced
Today our 2021 Biotech Pick of the Year Dimerix (ASX:DXB) announced the results from its low-cost investigator-led COVID-19 study, CLARITY 2.0.
This was the first time that DXB’s treatment, DMX-200, was used to treat respiratory conditions, which is a very different cohort of patients compared to kidney disease.
The most important takeaway for us is DMX-200 was found to be generally safe in patients with respiratory conditions, adding to the growing strong safety profile of the treatment.
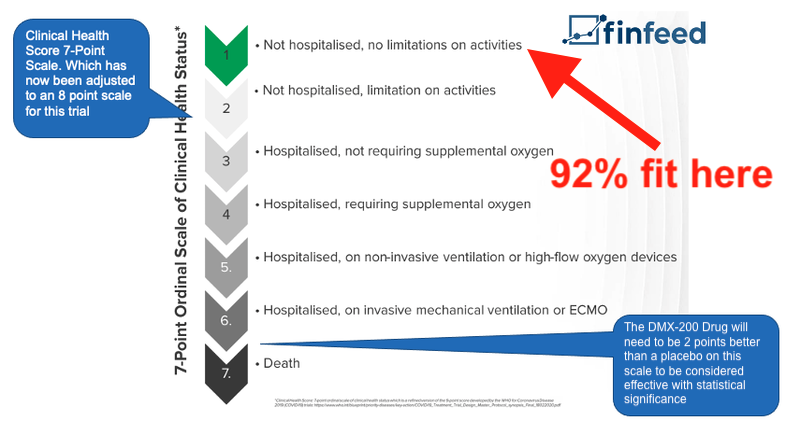
With regards to efficacy, 92% of patients scored a “1” on the Health Score Scale - this number includes both DMX-200 and placebo patients.
It is difficult for DXB to attribute these strong results to the DMX-200 treatment with any statistical significance, as there was a limited number of patients recruited into the trial (49) and the patients for the most part were young (median age 37) and vaccinated.
Our attention now turns to DXB’s main Phase III clinical trial for FSGS (a rare kidney disease), where DXB is 90% of the way through to recruitment for the interim analysis data (at 72 patients).






